For both cannabis consumers and companies, chalk it up to a lesson learned. The vaping crisis officially kicked off on August 23, 2019, when Illinois reported the first vape-related death. After a rash of hospitalizations and deaths attributed to vaping, the FDA launched a criminal investigation in September. Now it’s high time for the marijuana industry to earn back the trust of consumers — and regain lost sales (and revenue) with the help of technology.
The aftermath of the crisis means that consumers want to know what’s in their cannabis products, and that’s where tech solutions come into play. There are three primary methods that companies can use right now to build trust and maintain transparency – smartphone technology, encryption and enhanced testing. Let’s explore what that means for you.
Smartphone tech
Android operating systems have a plethora of apps available to help drive consumer confidence. While Apple has banned all vaping apps, those using Android platforms can request and receive certificates of analysis (COA) and test results.
Pax Labs, a marijuana vaporizer company based in San Francisco, was already actively developing a smartphone app (called the POD ID system) for consumers before the vape crisis. They rolled it out in November. The strategy is to give consumers access to information and, in essence, prove to the customer that they are purchasing a safe product. In addition to the COA and state testing results, the app includes information about the producer, the strain, potency and reviews. The app allows consumers to purchase a vape cart (the pod) from a dispensary and receive the lab report and more, all at the point of sale.
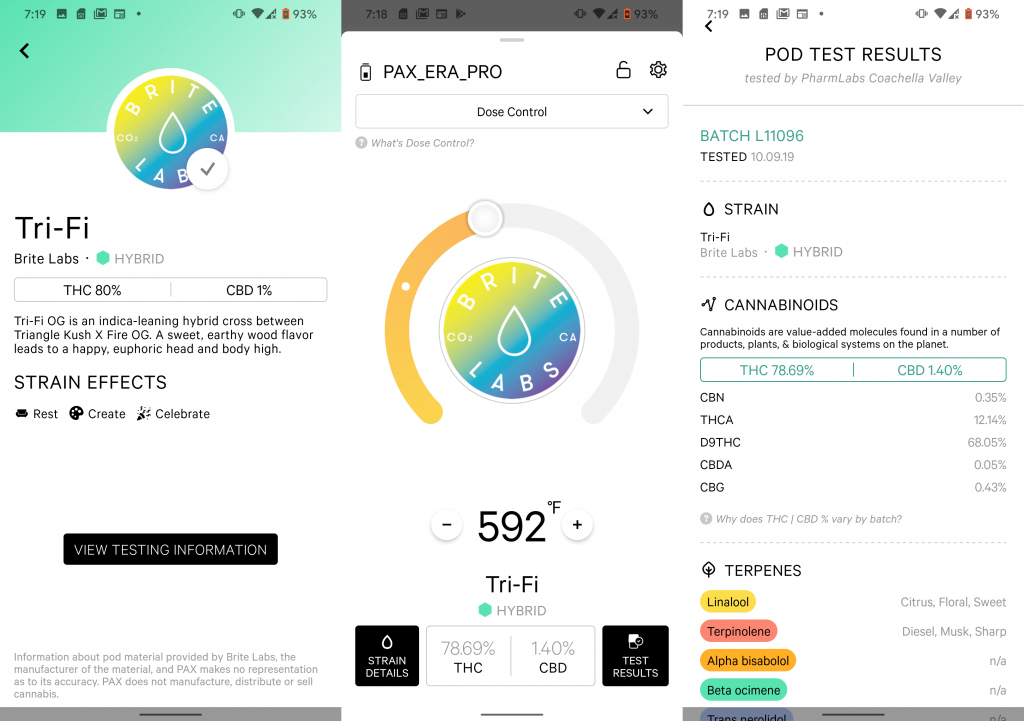
There are also apps utilizing QR codes to assist consumers with dosage and usage monitoring. Another company, Lucid Green, offers the consumer the Lucid ID app, which is designed to give immediate access to information about cannabis vape products. Scan the QR code (called the Lucid ID) with your smartphone and retrieve state-approved lab results, ingredients, dosage guidelines, strain effects and consumer product reviews. Budtenders can also scan products with the QR codes to assure customers that the product has had extensive testing.
Black market vape products are less likely to have these specialized features, and that can be the first line of defense in avoiding future health scares involving unregulated products and ingredients.
Encryption
Legitimate vape manufacturers are turning up the heat on the packaging to help distinguish the quality goods from their illicit counterparts, many of which look just like they came from licensed cannabis companies. From barcodes and microchips to security labels, the game aims to steer consumers far away from the black market products that many believe are the source of the illnesses and deaths associated with the vape crisis.
One company, Airgraft, based in Montreal, manufactures vape oil pods that contain an encrypted chip that, in essence, “talks” to the vaping device, and only legal, legit pods can be used with the device. Each pod is digitally linked to a specific batch of vape oil. When the user activates the device, the chip confirms the vape oil is legitimate. If it’s not, you can’t consume the oil. The Airgraft app also allows consumers to access the pod’s test results, COA and ingredients.
Another heavy hitter, KushCo Holdings, a marijuana company based in California, joined with De La Rue, a company that prints and distributes over 8 billion product authentication labels per year. The collaboration will give companies more choices to set their products apart with enhanced packaging options. Using 3D photopolymer images, counterfeiting just became more complicated, with secure packaging options that include secure visual authentication technology, unique serialization, e-verification, label-tracking and data-capturing features.
Enhanced testing
Some vape companies take things one step further in their supply chain, only working with specific producers and maintaining strict protocol on what ingredients are — and aren’t — allowed in their products while also mandating additional testing to ensure standards are met.
Some companies refuse to allow any cutting agents, like vitamin E acetate, in vape cartridges. Vitamin E acetate is one of the specific ingredients that the FDA has identified as a likely source of illness related to the vape crisis. As a result, consumers are demanding more and more information about just what’s in their vape cartridges, and companies need to be able to prove to the customer that their products are safe.
Some companies perform more stringent tests than what the state regulatory agencies require. For example, Seattle-based cannabis extraction company Lucid Oils goes above and beyond with their testing protocol. Their cannabis oils are tested first for mycotoxins and microbial contamination. Then, to ensure quality throughout, they test the oil in its cartridges once it’s been inserted into the hardware to check for any potential heavy metal leaks pre-purchase.
While these additional precautionary measures can add up to significant expenses for a company, the peace of mind far outweighs the cost. The vape crisis was bad for business in every adult-use marijuana market in the U.S., and while vape product sales are climbing, they are not back to pre-crisis levels. These precautions only emphasize the need for enhanced transparency and quality measures.